|
Breaking the titanium atom in its component elements
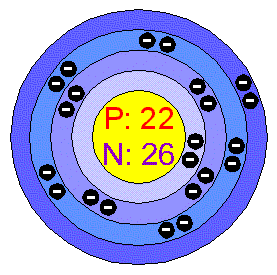
Titanium Atom Structure Atomic Radius: 2Å Atomic Mass Average: 47.876 AMU Electron Configuration: 1s2 2s2p6 3s2p6d2 4s2 Electrons per Energy Level: 2, 8, 10, 2 Shell Model
Filling Orbital: 3d2 Number of Electrons (with no charge): 22 Number of Neutrons (most common/stable nuclide): 26 Number of Protons: 22
Atomic radius is an indication of the sheer size of the titanium atom. You know, that atoms are basically spheres. Å is the abbreviation for angstrom, used to measure very, very small things. An angstrom is one ten-billionth of a meter (10-10m or 0.0000000001 m) or 1/10th of a nanometer. A typical atom is usually about one angstrom across, so that makes titanium atom pretty sizeable as compared to its neighbors in the periodic table of elements. Atomic mass average is an indication of the atom weight. Usually, the weight of an atom is determined by the number of neutrons and protons that are present in the nucleus and is expressed in AMU. AMU is the abbreviation for atomic mass unit, also called unified atomic mass unit or Dalton (Da) and it is the approximate mass of a hydrogen atom, a proton, or a neutron (1 AMU). The proton and neutron, which are similar in mass, each weighs approximately 1,836 times greater than a single electron, thus the mass contributed by electrons is insignificant when determining atomic weight or atomic mass. The atomic mass is the sum of the protons and neutrons in the nucleus. If you look lower in the sheet you will notice that titanium atom has 22 protons and 26 neutrons that will intuitively sum up to an atomic mass of 48. However, there are no less than 5 stable isotopes of titanium 46Ti, 47Ti, 48Ti, 49Ti, 50Ti, with 24,25,26,27,and 28 neutrons respectively, 48Ti being the most widespread stable isotope. The Atomic Mass Average is the sum of all isotope masses multiplied by their natural abundance. This weighted average is the relative mass listed in the periodic table of elements. That’s about all there is to say about the center of the atom, the nucleus. Let’s look at the other parts, more precisely, at the electron cloud. In an atom, the electrons spin around the nucleus. The electrons like to be in separate shells, although the term was replaced by the term energy levels, as the word shell suggests a fixed position, which is far from reality. We will use energy level to describe the possible location of electrons.
The electrons don't stay in defined areas around the nucleus. They are found in clouds that can have different shapes that include spheres and dumbbell-like shapes. There are seven energy levels. Each has a specific maximum number of electrons that can exist in it. The number of electrons, which an energy level can hold is equal to 2n2 where n = energy level. The letter n represents the principal quantum number that specifies the energy level of the atom in which an electron is located. The chart below identifies the various energy levels and maximum number of electrons possible. The energy level closest to the nucleus is represented by energy level 1.
Alternatively, you could download a completely FREE e-book outlining the defining aspects of titanium metal !!! Return from Titanium Atom to Titanium Element Return to Titanium Home Page
|